Post Market Surveillance (PMS) for Medical Devices
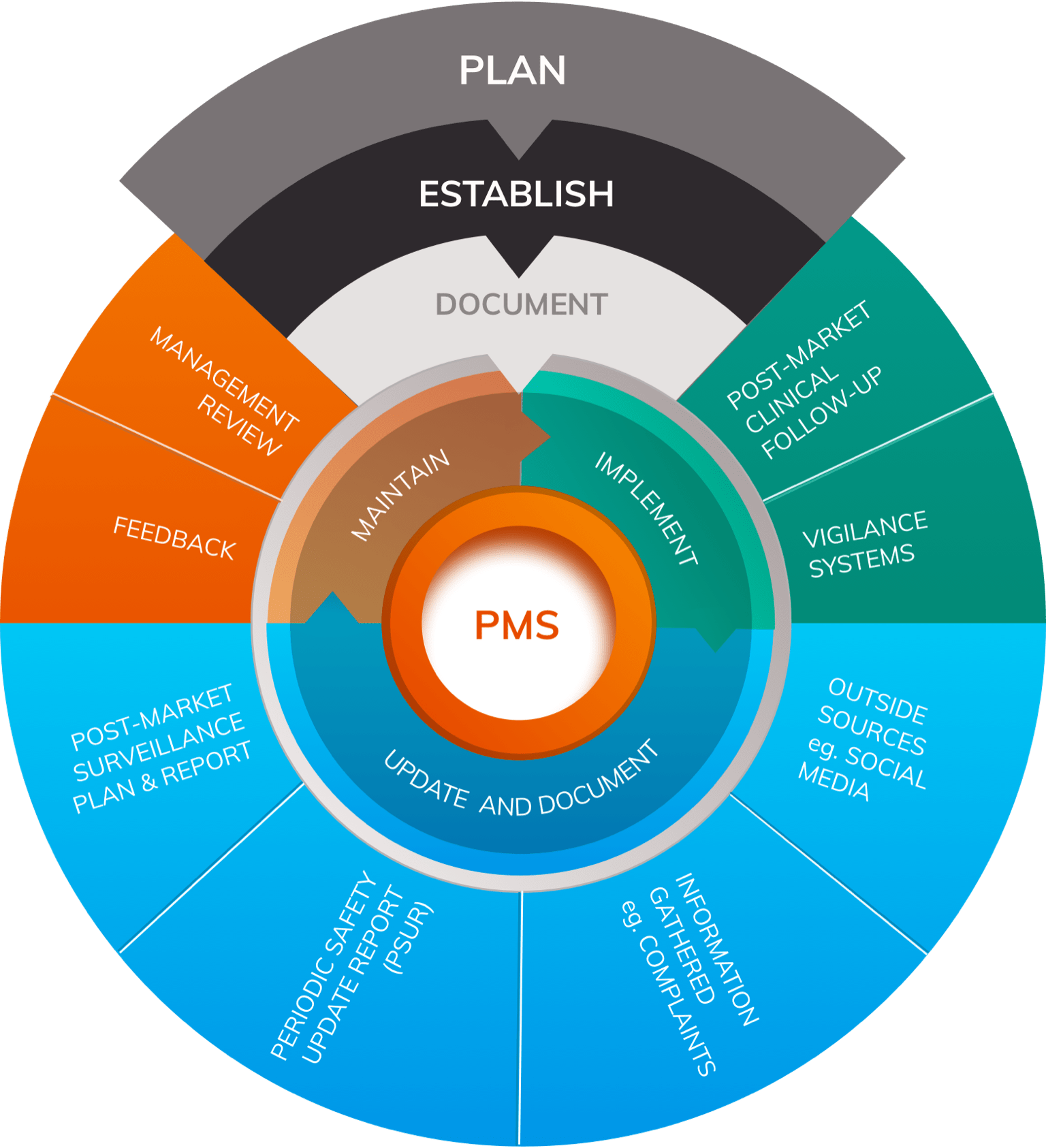
Post Market Surveillance is undertaken as a responsibility of the manufacturer it is different from market surveillance which is used to describe activities to monitor compliance with the Regulations undertaken by and coordinated between national competent authorities.
1. Class I devices are typically exempt from post market surveillance requirements
2. PMS need to carry out for Class II and Class III device
3. Proactive and systematic allows cooperation on vigilance and market surveillance
4. Connects with corrective action or preventive action processes
5. Allows update of technical documentation, including the risk-benefit determination and clinical evaluation/performance evaluation
6. Part of the manufacturer’s QMS
PMS activities involves following steps:
1. Defining Post-marketing surveillance objectives
2. Surveillance planning
3. Data collection, receipt and pre-analysis
4. Analysis of surveillance data
Post PMS analysis, the results could lead to:
1. Corrective and preventive action management
2. Communication with customers to improve the device performance
3. Change in the intended use





