Pre-Market Submission 510(k)-US FDA

Pre-Market Submission 510(k) must demonstrate that the device is substantially equivalent to one legally in commercial distribution in the United States: (1) before May 28, 1976; or (2) to a device that has been determined by FDA to be substantially equivalent. There are three different types of 510(k) submissions.
a. Special 510(k): This type of submission can be done to the FDA only if there is a modification to the already 510(k) cleared.
b. Abbreviated 510(k): If the FDA has recognized guidance specific to the device classification, then the manufacturer has to submit an abbreviated 510(k).
c. Traditional 510(k): If the submitter is submitting for a new device or modified device which requires more than 1 functional area of expertise
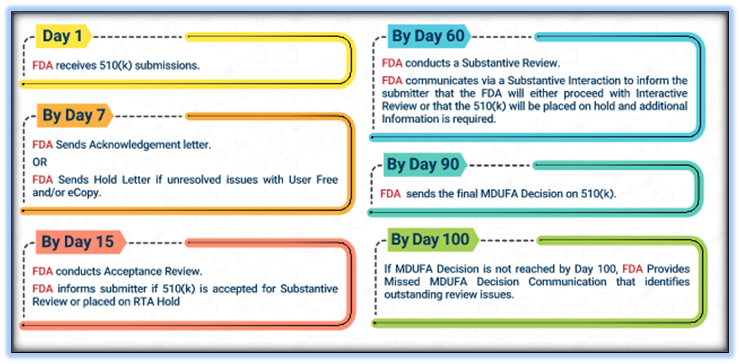
Timeline of communication during 510(k) Review:
Generally, 510(k) applicants can expect submission acceptance review decisions within 15 calendar days; substantive review decisions within 60 days; and final decisions within 90 days. Applicants with outstanding review issues will be notified within 100 days.