What is Software As a Medical Device?
Software will dominate the medical device market in the near future as technology develops and becomes more and more embedded in digital platforms, where its applications in the healthcare and medical sectors are particularly prominent.
The IMDFR defined SaMD as a “software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device”.
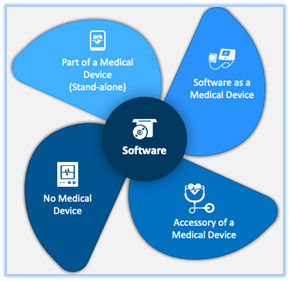
There are three types of software related to medical devices
- Software as Medical Device (SaMD)
- Software that is integral to a Medical Device
- Software used in the manufacture or maintenance of Medical Device
Examples of SaMD:
- Software that allows a mobile device to view images from an MRI, ultrasound, or X-ray that are used for diagnostic purposes
- Software that processes images which help to detect breast cancer
Classification of SaMD:
SaMD is categorized into class I to IV based on the levels of impact on the patient or public health and also the level of risk with class I represent the low risk and class IV with high risk.
General Considerations for SaMD:
IEC 62304 is a standard for life-cycle development of medical device software. The standard specifies a risk-based decision model, defines some testing requirements, and highlights three major principles that promote safety relevant to SaMD.





